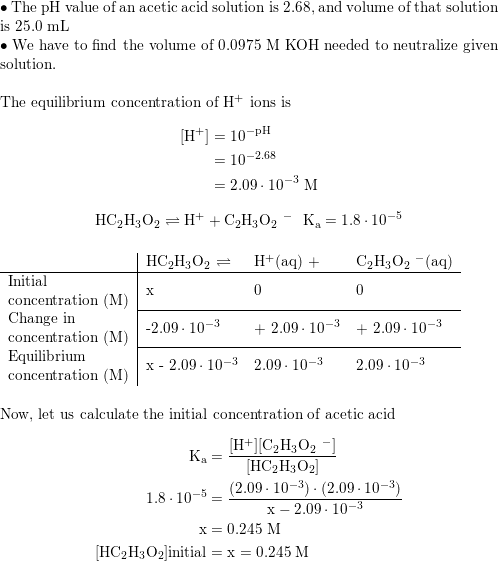
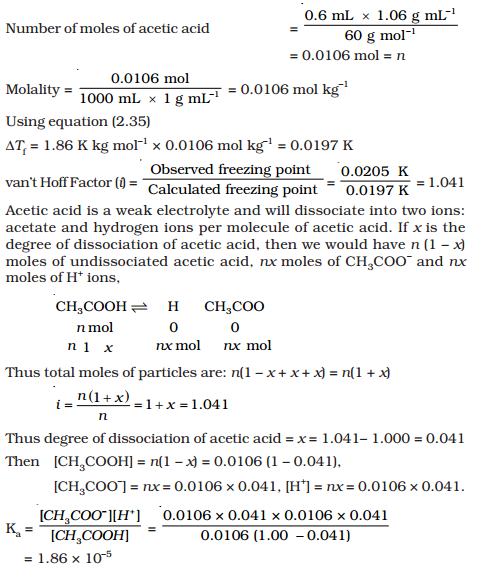
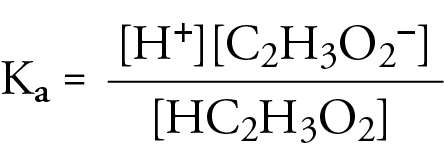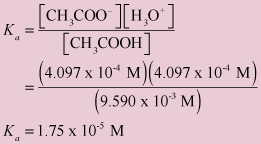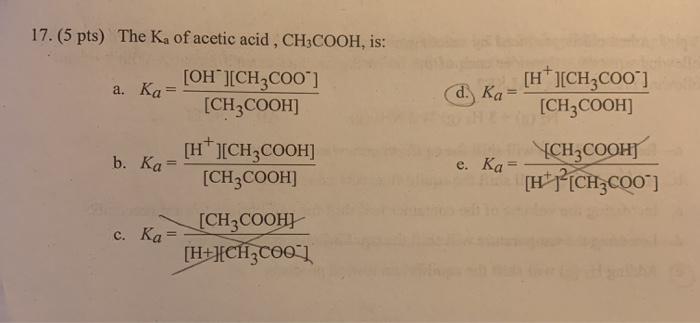Acetic acid partitioning as a function of pH, shown as the fraction of... | Download Scientific Diagram

How To Calculate the PH of a Buffer Solution | Equation & Example - Video & Lesson Transcript | Study.com
THE DISSOCIATION CONSTANT OF ACETIC ACID FROM 0 TO 35° CENTIGRADE1 | Journal of the American Chemical Society

Ka for CH3COOH is 1.8×10^-5. find out the % dissociation of 0.2M CH3COOH in 0.1M HCl solution? - EduRev NEET Question

The ionization constant of acetic acid `1.74xx10^(-5)`. Calculate the degree of dissociation of ... - YouTube
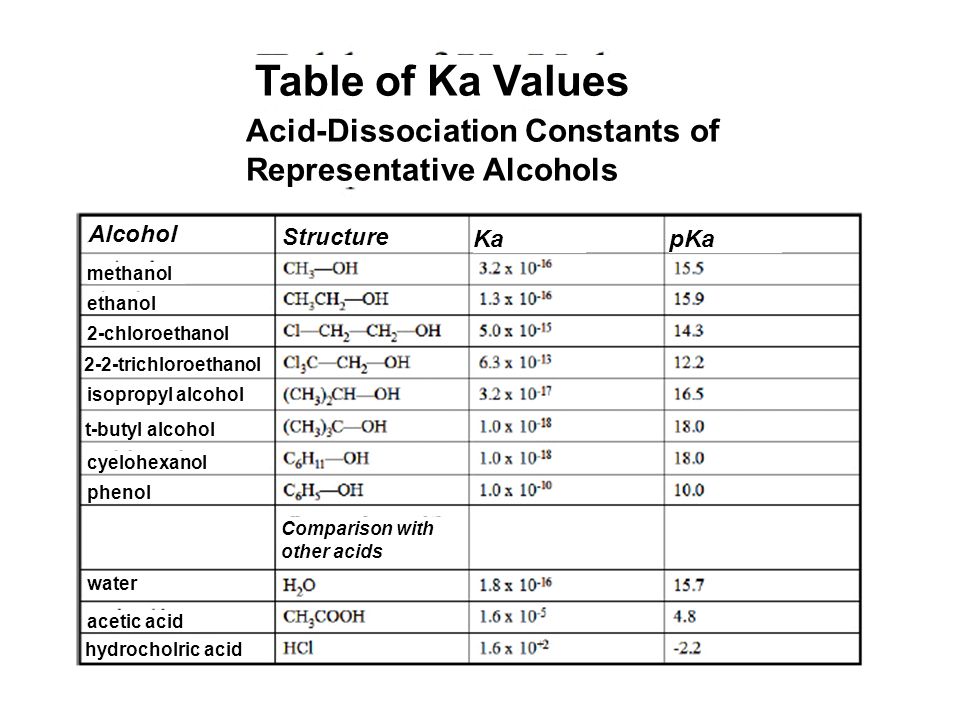
Table of Ka Values Acid-Dissociation Constants of Representative Alcohols Alcohol Structure Ka pKa methanol ethanol 2-chloroethanol 2-2-trichloroethanol. - ppt video online download

A 0 05 n sol of acetic acid is found to be 1 9+ ionised at 25 c calculate - Chemistry - Equilibrium - 13276973 | Meritnation.com

A student prepared 0.10M acetic acid solution and experimentally measured its pH to be 2.88. Calculate Ka - Brainly.in

What is the pH of a 10 mM solution of acetic acid (CH3COOH)? Acetic Acid Ka= 1.76 x 10^{-5} M. | Homework.Study.com
![What is the pH of a 1 M CH3COOH solution? [ Ka of acetic acid = 1.8 × 10^-5, Kw = 10^-14 mol^2 litre^-2 ] What is the pH of a 1 M CH3COOH solution? [ Ka of acetic acid = 1.8 × 10^-5, Kw = 10^-14 mol^2 litre^-2 ]](https://i.ytimg.com/vi/5MXjDjLyUp4/maxresdefault.jpg)