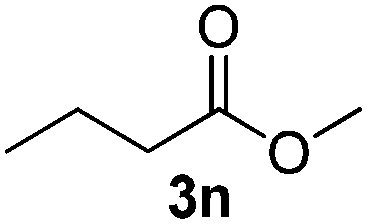
Amberlyst-15 catalysed oxidative esterification of aldehydes using a H 2 O 2 trapped oxidant as a terminal oxidant - New Journal of Chemistry (RSC Publishing) DOI:10.1039/C6NJ03831J

Recent advances in catalytic synthesis of 2,5-furandimethanol from 5-hydroxymethylfurfural and carbohydrates | Bioresources and Bioprocessing | Full Text
![Experimental and Simulation Studies for Purification and Etherification of Glycerol from the Biodiesel Industry[v1] | Preprints.org Experimental and Simulation Studies for Purification and Etherification of Glycerol from the Biodiesel Industry[v1] | Preprints.org](https://www.preprints.org/img/dyn_abstract_figures/2023/10/e3e9e656610d9988dac2924cfa833764/Screenshot_50.png)
Experimental and Simulation Studies for Purification and Etherification of Glycerol from the Biodiesel Industry[v1] | Preprints.org
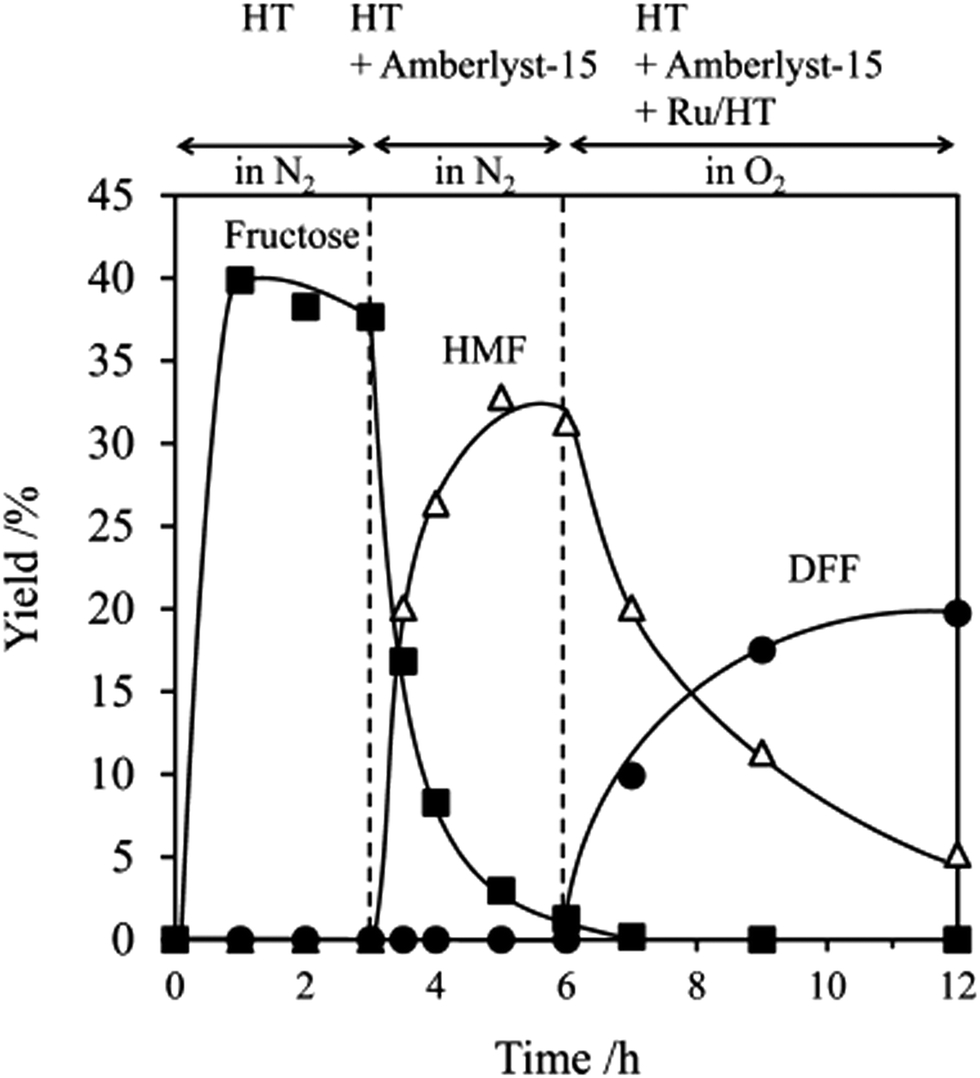
Catalytic oxidation of carbohydrates into organic acids and furan chemicals - Chemical Society Reviews (RSC Publishing) DOI:10.1039/C7CS00213K